
Effects of radiation
- Home
- Effects of radiation
What is Radiation?
Ionizing radiation is produced when radioactive particles, which are particles that are energetically unstable due to the numbers of protons and neutrons being unbalanced, need to release energy in order to reach stability. Radiation generally refers to ionizing radiation that is categorized into 2 types: particle beams, such as alpha (α) rays and beta (β) rays, and electromagnetic rays, such as gamma (γ) rays and X-rays. Some forms of electromagnetic waves such as electric waves, infrared rays, and visible rays, do not cause ionization and are called nonionizing radiation.
The ability for a particle to release radiation is called “radioactivity,” and materials with such ability are called “radioactive materials.” To illustrate this with a flashlight, the light is radiation, the flashlight-device is a radioactive material, and its ability to emit light is radioactivity. Just like how your body receives light but does not begin emitting it, when your body is exposed to radiation, it does not become radioactive. When radiation is detected on your body it is due to radioactive particles attached to your skin or clothing. The radiation will be gone once the radioactive substances have been removed, which can be done by washing, etc. Radiation is not contagious, and does not get passed around from person to person.
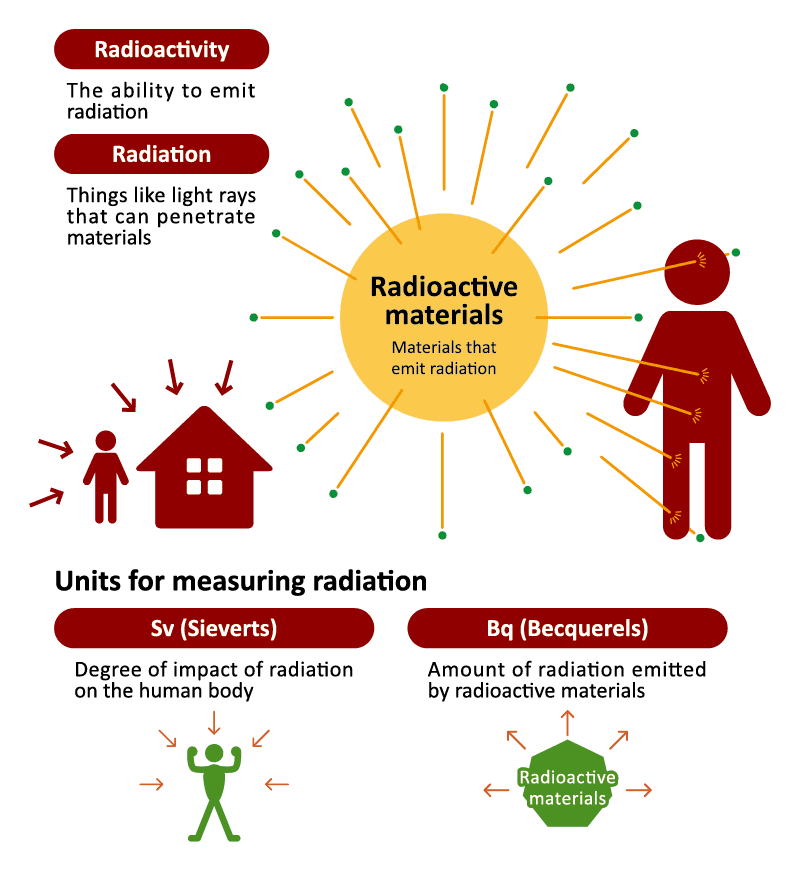
What do the units Sv and Bq mean?
Units of radiation can be divided roughly into units for measuring radiation that is given off, and units for measuring radiation that is exposed. A “Becquerel (Bq)” is a unit used for measuring the intensity of radioactivity and is for measuring radiation that is given off. Units such as “gray (Gy)” (used for absorbed dose) and “Sievert (Sv)” (used for equivalent dose, effective dose and ambient dose, etc.) are for measuring radiation that has been received or exposed.
For this reason, a Bq is used when indicating the ability of radioactive materials to emit radiation contained in food, water or other substances. It is therefore used together with a unit for mass such as Bq/Kg and Bq/L.
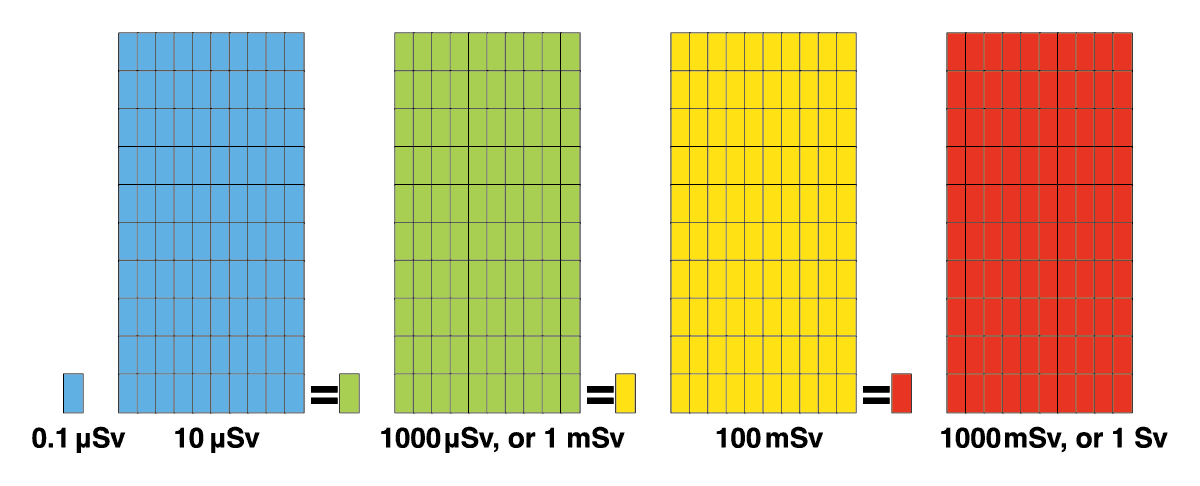
The Sv is indicates the effects of such radiation on the human body. Radiation of identical Sievert values will always have the same level of effect on the human body, regardless of it being from a natural or artificial source of radiation. Since the Sv is a very large unit, you often see the unit μSv (micro Sievert, or one millionth of a Sv) or mSv (milli Sievert, or one thousandth of a Sv) being used, and in combination with a unit for time, such as μSv/h or mSv/year.
1 Sv = 1000mSv Bq / Kg = Bq per kilogram
1 mSv = 0.001 Sv, or 1000 μSv Bq/ l = Bq per liter
1 μSv = 0.001 mSv
Often seen as:
μSv / hour = the amount of radiation you receive if you stay there for one hour
mSv/ year = the amount of accumulated radiation you receive in one year at that spot
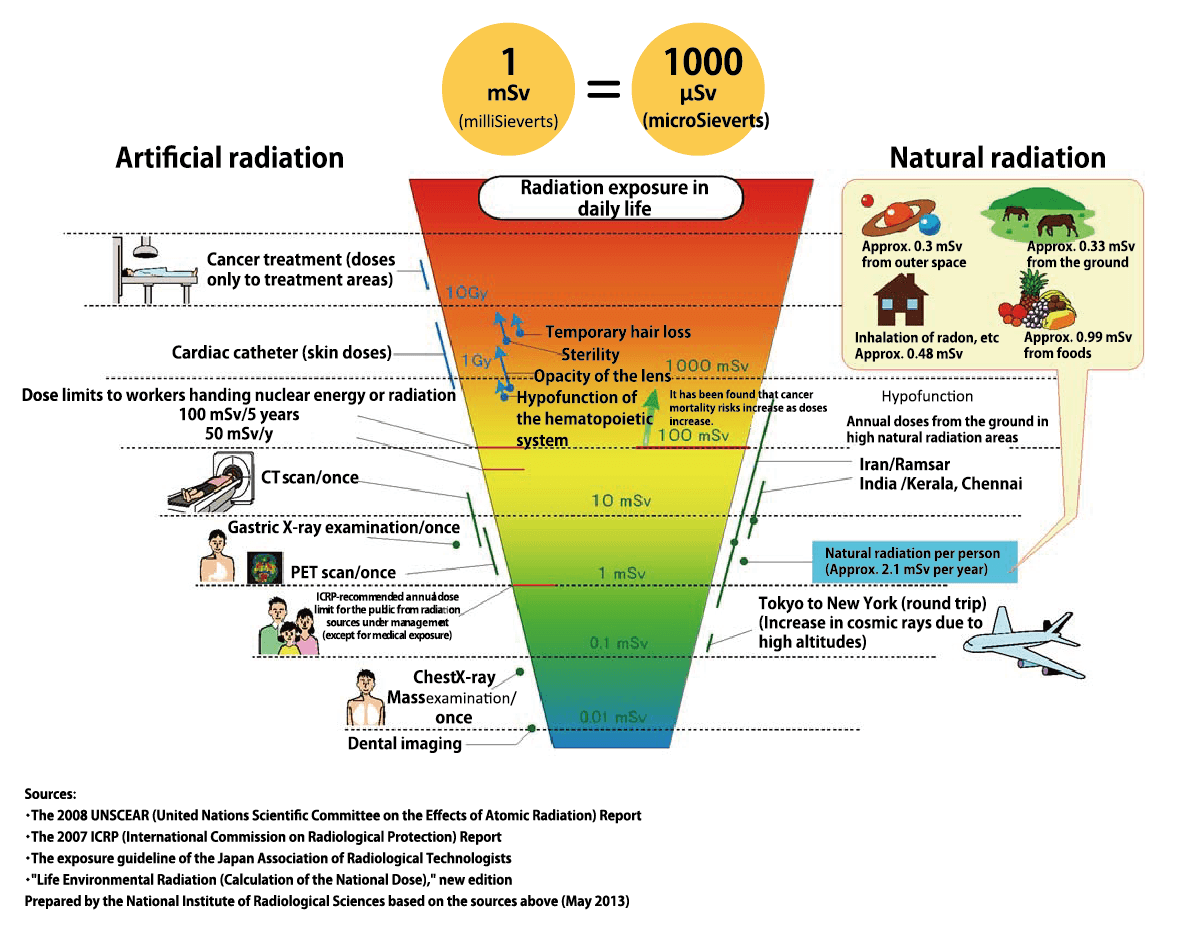
Radiation doses in everyday life
mSv: millisieverts
Are Radiation levels in Fukushima still considered high ?
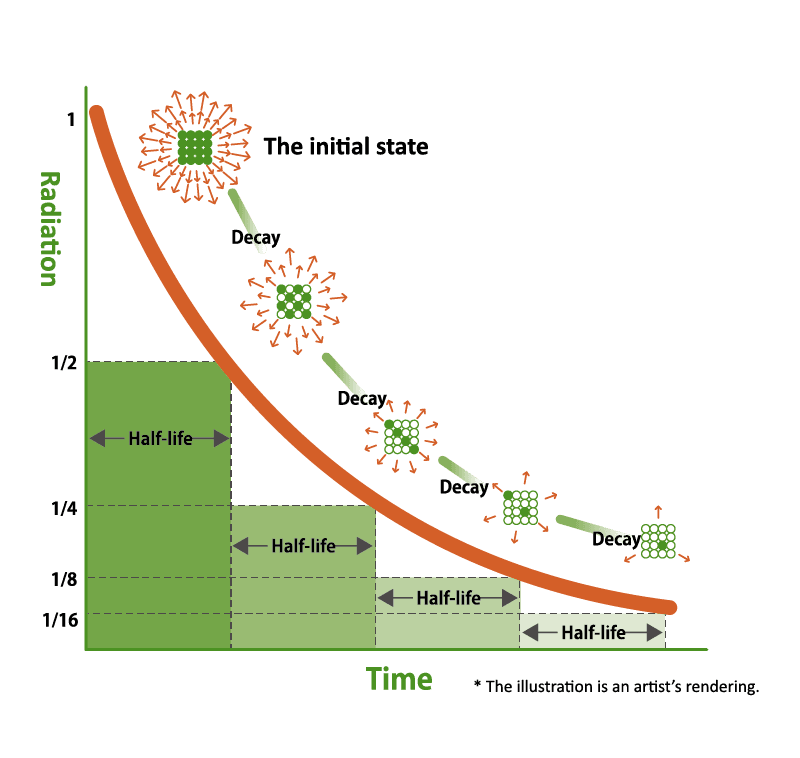
Radiation levels throughout Fukushima have decreased over time due to a variety of factors. The biggest factor is the physical half-life of radioactive nuclides. Radioactive materials turn into stable particles that no longer release radiation over time. The amount of time required for radioactive materials to be reduced by half is called the “physical half-life.” The physical halflife of radioactive materials depends on the type of material, and in Fukushima’s case, the physical half-life of the three most common radioactive nuclides Iodine 131, Cesium 134, and Cesium 137, are 8 days, 2 years, and 30 years, respectively. This, in addition to decontamination work that was conducted throughout the prefecture to speed up this process, has led to a significant drop in radiation levels, to the point where Fukushima’s levels are now comparable to levels found around major cities in the world.
How much Radiation is dangerous?
First and foremost, it is important to understand we are constantly exposed to a small amount of radiation no matter where we live or what we do. This is because radioactive substances exist around the world naturally. The effects of radiation therefore depend not on its “presence” but the “amount” of exposure. When our body is exposed to radiation, its energy may damage some DNA (genes) in our cells. However, because our body has enzymes that repair such damage, and can also replace unrepaired cells with healthy cells through the process of metabolism, small amounts of radiation exposure do not have any significant impact on our health.
On the other hand, exposure to a high dose of radiation at once may result in our body’s DNA repair mechanisms being unable to keep up with radiation damage, therefore causing health hazards. According to the ICRP (International Commission on Radiological Protection) these begin to occur after acute exposure exceeds a threshold value of around 0.1 to 0.5 Sv (or 100,000 μSv to 500,000 μSv). Furthermore, according to an epidemiological survey on survivors from the Hiroshima and Nagasaki atomic bombings, it has been agreed that after cumulative exposure doses exceeds around 100 to 200 mSv, the risks of cancer mortality increases as the exposure doses increases.
At the same time, current scientific evidence suggests that the impact of exposure to radiation of less than 100 mSv is too small for us to detect any significance. This is so because cancer-causing factors other than radiation found in various aspects of our life including stress, drinking and smoking, have larger probability of causing cancer mortality than radition exposure of less than 100mSv.
Furthermore, it is important to know that in regards to inheritable characteristics, according to all past research including the atomic bomb cases, there have been no proven cases of radiation exposure resulting in certain characteristics being inherited down generations. Furthermore, there have been no rises in rates of birth defects among children who were fetuses at the time of the Fukushima Daiichi Nuclear Accident.
Risks of Cancer (Radiation)
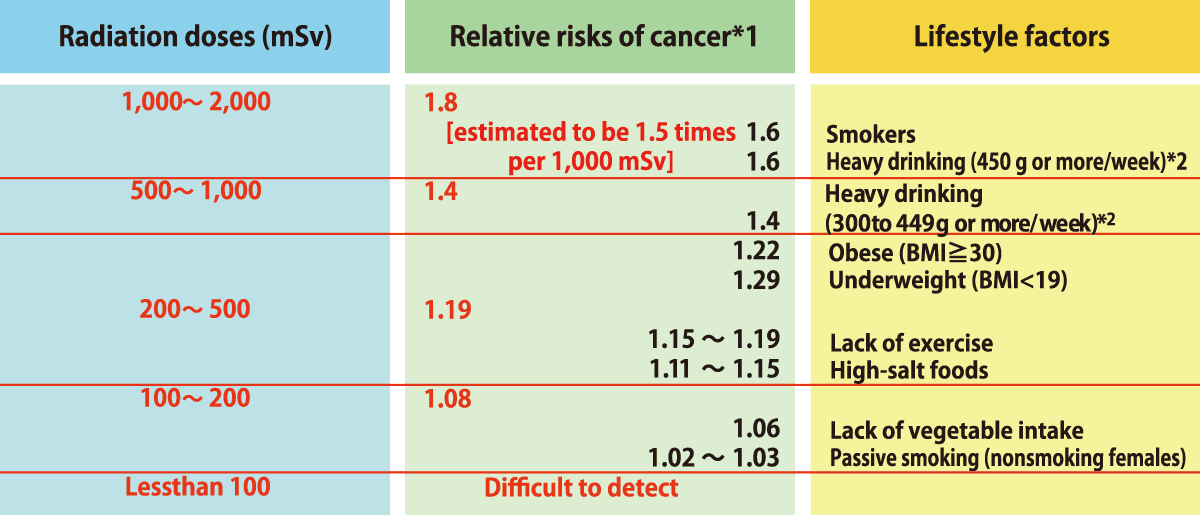
- *1
Risks of developing radiation-induced cancer are based on the data (solid cancers only) obtained from the analysis of instataneous exposure due to the atomic bombing in Hiroshima and Nagasaki, and are not based on the observation of long-term exposure effects.
- *1
Relative risks indicate how many times larger the cancer risks are among people exposed to radiation when assuming the risks among non-exposed people as 1.
- *2
Alcohol consumption is in ethanol equivalent.
Source: Website of the National Cancer Center Japan
What are the differences between Fukushima and Chernobyl accidents?
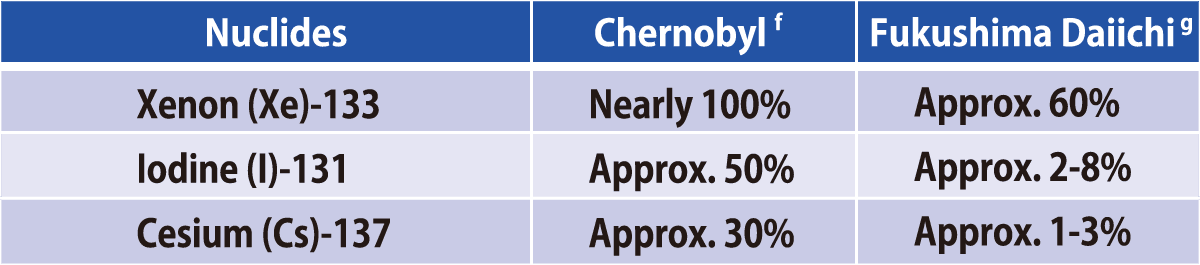
With regard to the Chernobyl Nuclear Accident (April,1986), there was a much larger release of radioactive materials compared to Fukushima including Iodine, Strontium, and Plutonium. Furthermore, food and drinks consumed by those living in affected areas were mainly self-grown, and internal exposure from the intake of contaminated food, especially the intake of milk contaminated with radioactive iodine, increased radiation doses in their thyroid glands. According to the UN, due to prompt measures not taken in Chernobyl, thyroid cancer was observed in more than 6,000 children and youths who had consumed radioactive iodine-131, of which 15 have lost their lives as of 2005.
On the other hand, in Fukushima’s case, there were very small releases of strontium and plutonium, and iodine that was released disappeared in the early stages. For this reason, as of January 2016, government surveying has mainly focused on the influence of radioactive cesium. Japan also was quick to implement evacuation orders, and placed shipping restrictions on agricultural products, resulting in very minimal amounts of radioactive materials being consumed by residents. It also set strict benchmark values for radioactivity, and systems to conduct vigilant and watchful inspections to make sure foods exceeding the benchmark values will not be sold in markets. As a result, any internal exposure dose has been maintained at levels much lower than 1 mSv.
A preliminary report from the World Health Organization (WHO) in 2013 estimated the radiation doses that residents of Japan outside the evacuated areas received in the year following the accident, and concluded that most people in Fukushima prefecture would have received a radiation dose of between 1 and 10 mSv during the first year after the accident. This is in addition to levels of about 2.4 mSv they would have received from unavoidable natural sources. The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) also carried out a realistic dose assessment in 2016 and drew upon the following conclusions:
- No acute health effects resulting from the Fukushima Daiichi Nuclear Accident can be observed
- The occurrence of a large number of radiation-induced thyroid cancers in Fukushima Prefecture – such as observed after the Chernobyl accident – can be discounted because absorbed doses to the thyroid after the FDNPS accident were substantially lower than those after the Chernobyl accident.
- The prenatal exposure resulting from the accident at FDNPS is not expected to increase the incidence of in spontaneous abortion, miscarriages, perinatal mortality, congenital effects or cognitive impairment prenatal exposure.
Both reports agree the most serious health effects of the nuclear accident in Fukushima in the short term appear to be on mental and social well-being. This is further proven by the fact that in-direct casualties in Fukushima are now larger than the numbers of those who lost their lives due to direct damages. Furthermore, the evacuation following the accident caused immediate aggravation of the condition of already vulnerable groups.
Comparison of Estimated Amounts of Released Radionuclides
between Chernobyl and Fukushima Daiichi NPS Accidents
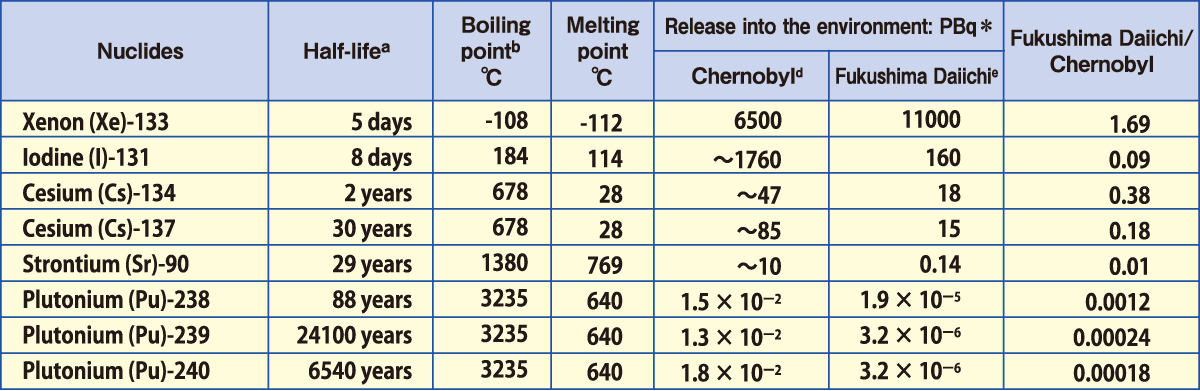
of radionuclides accumulated in the reactor core at the time of the
accidents that were released into the environment
*PBq equals 1015Bq.
Sources:a:ICRP Publication72(1996);b and c(except for Np and Cm):Rikagaku Jiten 5th edition(1998);d: UNSCEAR 2008
Report, Scientific Annexes C,D and E; e:Report of Japanese Government to the IAEA Ministerial Conference on Nuclear
Safety (June2011); f: UNSCEAR 2000 Report, ANNEX J; g:UNSCEAR 2013 Report,ANNEX A
-
Ministry of the Environment, Government of Japanhttps://www.env.go.jp/en/chemi/rhm/basic-info/
-
Consumer Affairs Agency, Government of Japanhttps://www.caa.go.jp/disaster/earthquake/understanding_food_and_radiation/material/pdf/130902_food_qa_en.pdf

